
by Dr. Yashashwini Reddy | May 5, 2025
The tablet compression machine is a vital piece of equipment used in the pharmaceutical industry to produce tablets in large quantities. It works on the principle of compressing powder or granules into a solid form using pressure. The working and principles of tablet...

by Dr. Yashashwini Reddy | May 3, 2025
Tablet Manufacturing Process: An Overview The tablet manufacturing process is a complex and precise procedure that transforms raw materials into the final pharmaceutical or nutraceutical product. The process involves several key stages, each critical to ensuring the...

by Dr. Yashashwini Reddy | May 2, 2025
The tablet manufacturing process is a complex and highly regulated series of steps that ensures the final product is safe, effective, and of consistent quality. Below is a detailed explanation of the key stages involved in tablet manufacturing: 1. Pre-Formulation...

by Dr. Yashashwini Reddy | Apr 15, 2025
Sugar coating is when a layer of sugar or sucrose is applied over the core tablet, mainly to mask the taste. It’s the oldest technique used in pharmaceutical industries, and even though it’s a multistage and lengthy process, it’s been trusted for years....
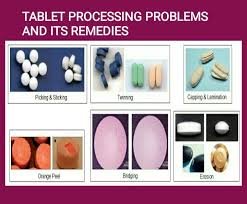
by Dr. Yashashwini Reddy | Apr 13, 2025
Tablet twinning refers to a manufacturing flaw in which two or more tablets adhere to one another, creating a “twin” or cluster during the coating phase. This issue is particularly prevalent among flat or parallel-faced tablets, primarily due to moisture...







