
by Dr. Yashashwini Reddy | Oct 8, 2025
🧪 ICH Q2: Analytical Validation 🔹 Full Title: ICH Q2(R2) – Validation of Analytical Procedures 🔹 Objective: To provide guidance on the validation of analytical methods used to test drug substances and products — ensuring that the methods are accurate, reliable, and...
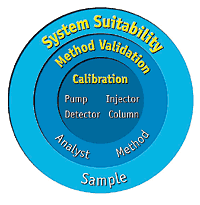
by Dr. Yashashwini Reddy | Aug 9, 2025
System Suitability in HPLC Analysis What is System Suitability? System Suitability Testing (SST) is a set of analytical checks performed before and during analysis to ensure that the HPLC system and method are capable of producing accurate, precise, and reproducible...

by Dr. Yashashwini Reddy | Apr 29, 2025
System suitability in High-Performance Liquid Chromatography (HPLC) analysis is a set of tests conducted to ensure that the HPLC system is performing optimally for the specific analysis being performed. These checks help determine if the system is capable of producing...

by Dr. Yashashwini Reddy | Apr 21, 2025
Standard Operating Procedure (SOP) Here is a Standard Operating Procedure (SOP) for the Verification of System Suitability Test (SST). This procedure ensures that analytical systems perform according to predefined specifications before performing...







