
by Dr. Yashashwini Reddy | Jun 25, 2025
✅ GVP Module VIII – Post-Authorization Safety Studies (PASS) 📘 Purpose GVP Module VIII provides guidance on the design, conduct, and reporting of Post-Authorization Safety Studies (PASS) conducted by Marketing Authorization Holders (MAHs) after a product has been...

by Dr. Yashashwini Reddy | Jun 18, 2025
Here’s a complete view of pharmacovigilance—a structured, end‑to‑end journey that ensures drug safety from lab to real-world use: 1. Definition & Scope Pharmacovigilance (PV) is the science and set of processes for detecting, assessing, understanding, and...

by Dr. Yashashwini Reddy | May 1, 2025
Quality by Design (QbD) in Pharmaceuticals: A Detailed Explanation Quality by Design (QbD) is a systematic approach to pharmaceutical development that aims to ensure the quality of a drug product through the design and control of the manufacturing process. This...
by Dr. Yashashwini Reddy | Apr 29, 2025
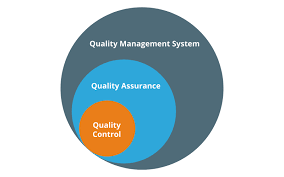
Supplier audits have a significant and multifaceted impact on quality assurance in the pharmaceutical sector. Here’s a detailed breakdown of their influence: 1. Ensuring Compliance with Regulatory Standards Supplier audits help ensure that raw material...
by Dr. Yashashwini Reddy | Apr 27, 2025
Standard Operating Procedure (SOP) 1. Purpose The purpose of this SOP is to provide guidelines for the identification, handling, storage, and disposal of prohibited items within [Organization/Department Name], ensuring compliance with...







