
by Dr. Yashashwini Reddy | Jun 25, 2025
✅ GVP Module X – Additional Monitoring 📘 Purpose GVP Module X outlines the principles and procedures related to “Additional Monitoring” of certain medicines to enhance early identification of new safety information. It ensures transparency and helps promote reporting...

by Dr. Yashashwini Reddy | Jun 25, 2025
✅ GVP Module VIII – Post-Authorization Safety Studies (PASS) 📘 Purpose GVP Module VIII provides guidance on the design, conduct, and reporting of Post-Authorization Safety Studies (PASS) conducted by Marketing Authorization Holders (MAHs) after a product has been...

by Dr. Yashashwini Reddy | Jun 18, 2025
Here’s a complete view of pharmacovigilance—a structured, end‑to‑end journey that ensures drug safety from lab to real-world use: 1. Definition & Scope Pharmacovigilance (PV) is the science and set of processes for detecting, assessing, understanding, and...

by Dr. Yashashwini Reddy | May 1, 2025
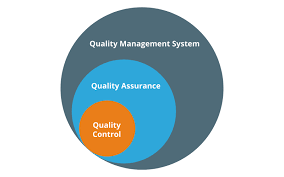
Quality by Design (QbD) in Pharmaceuticals: A Detailed Explanation Quality by Design (QbD) is a systematic approach to pharmaceutical development that aims to ensure the quality of a drug product through the design and control of the manufacturing process. This...
by Dr. Yashashwini Reddy | Apr 29, 2025
Supplier audits have a significant and multifaceted impact on quality assurance in the pharmaceutical sector. Here’s a detailed breakdown of their influence: 1. Ensuring Compliance with Regulatory Standards Supplier audits help ensure that raw material...







