by Dr. Yashashwini Reddy | Sep 15, 2025
GMP Audit Checklist – Storage of Starting Materials 1. Storage Area Design & Conditions Is the storage area clean, well-lit, pest-free, and secure? Are temperature and humidity continuously monitored and recorded? Are conditions maintained as per material storage...
by Dr. Yashashwini Reddy | Sep 9, 2025
🔐 Why Data Integrity is More Important Than Ever? 1. Patient Safety at the Core Medicines are only as safe as the data proving their quality. Any falsified, incomplete, or inaccurate record may lead to unsafe products reaching patients. Strong data integrity ensures...
by Dr. Yashashwini Reddy | Aug 30, 2025
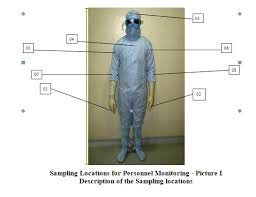
Standard Operating Procedure (SOP) for Microbiological Monitoring of Cleanroom 1. Purpose To describe the procedure for microbiological monitoring of cleanrooms to ensure controlled aseptic conditions and compliance with GMP guidelines. 2. Scope This SOP applies to...

by Dr. Yashashwini Reddy | Aug 18, 2025
Cleaning Validation of Manufacturing Equipment Cleaning validation is a documented process that ensures manufacturing equipment in the pharmaceutical industry is consistently cleaned to predetermined limits, preventing contamination and cross-contamination between...

by Dr. Yashashwini Reddy | May 1, 2025
Calibrating a refractometer is an essential process to ensure accurate readings. Here’s a detailed step-by-step guide on how to calibrate it: 1. Gather Materials Refractometer: Ensure it’s clean and in good condition. Calibration Solution: You will need a...





