by Dr. Yashashwini Reddy | Sep 18, 2025
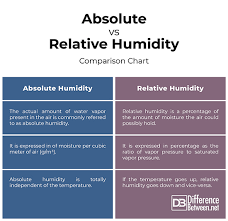
📌 Difference between Humidity and Relative Humidity 1. Humidity Definition: The amount of water vapor present in the air at a given time. Measurement: Usually expressed in grams of water vapor per cubic meter of air (g/m³). Type: Known as Absolute Humidity....

by Dr. Yashashwini Reddy | May 15, 2025
Standard Operating Procedure (SOP) Here is a detailed Standard Operating Procedure (SOP) for Entry and Exit into the Form Fill Seal (FFS) Area, commonly required in aseptic and controlled environments like pharmaceutical manufacturing:...

by Dr. Yashashwini Reddy | May 3, 2025
In a sterile facility, especially in industries like pharmaceuticals, biotechnology, or medical device manufacturing, maintaining a controlled environment is crucial for ensuring product safety and preventing contamination. One key component in maintaining sterility...





