Ensuring Cleanliness and Integrity of Buffer Areas in Controlled Sterile Settings.

In a sterile facility, especially in industries like pharmaceuticals, biotechnology, or medical device manufacturing, maintaining a controlled environment is crucial for ensuring product safety and preventing contamination. One key component in maintaining sterility is the buffer area. This area plays a vital role in controlling environmental conditions and acts as a barrier between controlled sterile environments and less controlled spaces. Here’s a detailed explanation of buffer areas and their maintenance in a sterile facility:
What is a Buffer Area?
A buffer area is a controlled environment within a sterile facility that helps to ensure the integrity of a clean or sterile space. These areas are typically located between cleanrooms (where sterile processes occur) and other parts of the facility, such as less controlled environments or hallways. Buffer areas are designed to minimize contamination risks when materials, personnel, or products move between zones of different cleanliness levels.
Functions of the Buffer Area:
-
Transition Zone: It acts as an intermediary between the sterile area and the less sterile surroundings, limiting the introduction of contaminants from the outside.
-
Pressure Control: Buffer areas are often maintained at a higher air pressure than the surrounding areas. This helps ensure that any air exchange flows from the cleanroom into the buffer zone, rather than allowing contaminants from less sterile spaces to flow into the cleanroom.
-
Environmental Control: The buffer area ensures strict control over key parameters such as temperature, humidity, air quality, and particulate matter. This is especially important in pharmaceutical production or medical device manufacturing, where even the slightest contamination can lead to product defects or safety risks.
-
Personnel and Material Access: The buffer area serves as a point for gowning and de-gowning personnel, as well as the proper handling of materials entering and leaving the clean areas. This controlled access is crucial to maintain the sterility of the environment.
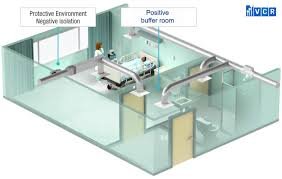
Maintenance of Buffer Areas:
Maintaining buffer areas in sterile facilities requires rigorous practices to ensure the environment stays contamination-free. Here’s a detailed look at how buffer areas should be maintained:
-
Air Quality and Filtration:
-
HEPA Filters: Buffer areas should be equipped with High-Efficiency Particulate Air (HEPA) filters that help remove airborne contaminants, including dust, microbes, and particles, which are critical to maintain the cleanliness of the surrounding sterile areas.
-
Airflow Management: The airflow in the buffer area is carefully controlled to prevent the ingress of contaminants. Laminar flow or unidirectional airflow is typically used to ensure that air moves in a consistent pattern, reducing the likelihood of particles entering from less controlled spaces.
-
-
Temperature and Humidity Control:
-
Maintaining a specific temperature and humidity range is important for the integrity of the processes happening in the adjacent sterile rooms. These environmental factors help ensure that conditions remain suitable for the products being manufactured (e.g., drugs or medical devices) while preventing microbial growth.
-
-
Pressure Differentials:
-
As mentioned earlier, buffer areas typically maintain a positive pressure relative to the surrounding environments. This helps prevent air from less clean areas from flowing into the sterile spaces. The air pressure must be continuously monitored to ensure it stays within the required parameters.
-
-
Cleaning and Sanitization:
-
Regular Cleaning: The buffer area must be cleaned regularly using disinfectants and approved cleaning agents. This includes both surfaces (walls, floors, counters) and air systems (ducts, vents, and filters). Cleaning schedules should be strictly followed to maintain sterility.
-
Wipe-down Procedures: Surfaces in the buffer zone are regularly wiped down to prevent dust and microbial build-up. Items that are brought into the area, such as equipment and supplies, must also be sanitized before entering.
-
-
Gowning and De-gowning Protocols:
-
Personnel Access: The buffer area is often used for gowning, where workers change into sterile garments before entering the cleanroom. This includes the use of gloves, face masks, hair covers, and protective suits. The gowning process is strictly monitored to prevent contamination.
-
Change of Garments: When personnel leave the sterile zone, they must change out of their sterile garments in the buffer area to avoid contaminating the surrounding spaces.
-
-
Material Handling:
-
Proper Storage: Materials, such as raw ingredients or equipment, entering the sterile area are often stored temporarily in the buffer zone. These materials are checked, sanitized, and prepared before being passed into the cleanroom. All materials must be verified for cleanliness and integrity.
-
Transfer Systems: In some facilities, items may be passed into the sterile environment via sealed systems like pass-through chambers or transfer hatches to prevent direct exposure to the outside air.
-
-
Monitoring and Documentation:
-
Environmental Monitoring: Continuous monitoring of air quality, temperature, humidity, and pressure is essential. Any deviations from the required parameters must be logged, and corrective actions must be taken immediately to restore the proper conditions.
-
Documentation and Compliance: Maintenance of the buffer area is highly regulated, and facilities must document all cleaning, maintenance, and monitoring activities. This documentation is often part of the regulatory requirements, such as Good Manufacturing Practices (GMP), enforced by bodies like the FDA.
-
-
Personnel Training:
-
Ensuring that all personnel are trained in sterile practices, including gowning, material handling, and proper hygiene, is essential for maintaining the integrity of the buffer area and the cleanroom environment. Periodic training and audits ensure ongoing compliance.
-
Conclusion:
The buffer area plays an essential role in the maintenance of sterile conditions within controlled environments. Its primary function is to act as a barrier that prevents contamination from entering sterile spaces, while also ensuring that any materials or personnel passing in or out are appropriately managed to maintain cleanliness. Rigorous maintenance, regular monitoring, and strict protocols for air quality, pressure, temperature, cleaning, and personnel behavior are essential for ensuring that buffer areas remain effective in their role. Proper maintenance of buffer areas is crucial to the success of the sterile facility’s operations and the safety of the products being produced.
🎓 Discover one of the best Pharmaceutical Production courses available — click below to explore the course that’s shaping future Production skills.

