The Role of Relative Response Factor (RRF) in HPLC and Its Determination.

The Role of Relative Response Factor (RRF) in HPLC and Its Determination-
High-Performance Liquid Chromatography (HPLC) is an essential analytical technique used in various fields like pharmaceuticals, environmental analysis, food testing, and biotechnology. It helps in the precise quantification of compounds in a mixture, often by measuring the area under the curve (AUC) of chromatographic peaks. However, to accurately quantify the concentration of a target compound, it’s crucial to account for differences in the detector’s response to different substances. This is where the Relative Response Factor (RRF) comes into play.
What is Relative Response Factor (RRF)?
The Relative Response Factor (RRF) is a correction factor used in HPLC to standardize the response of the detector for different analytes. Since detectors may respond differently to different compounds based on their chemical properties, the RRF compensates for these differences, ensuring that the quantification of compounds is accurate, even when the detector response varies.
The RRF is a ratio that compares the response of the analyte of interest to a standard (usually a reference compound) under the same experimental conditions.
Mathematically, the RRF is given by the following formula:
Where:
-
Response of analyte: The detector response (e.g., peak area or height) for the analyte.
-
Response of standard: The detector response for the standard substance (which should ideally be a compound of known concentration and similar chemical properties to the analyte).
-
Concentration of standard: The known concentration of the reference substance.
-
Concentration of analyte: The concentration of the analyte whose RRF is being calculated.
Importance of RRF in HPLC
-
Correct Quantification: Different compounds may have different molar absorptivity or chromatographic behavior, leading to variations in detector response. By using RRF, one can accurately convert the detector’s raw response into the concentration of the analyte.
-
Standardization of Data: RRF allows for the comparison of different compounds in a sample, ensuring that the concentration of any analyte can be determined based on a standardized method, even when different substances are being analyzed.
-
Method Validation: In quantitative HPLC analysis, calculating the RRF helps to validate the reliability and accuracy of the method, especially when different detectors or instruments are involved.
-
Improved Precision: The use of RRF increases the precision of results, particularly when the sample contains multiple compounds with varying responses to the detector.
Steps to Determine the Relative Response Factor (RRF)
To determine the RRF for an analyte, follow these steps:
1. Select an Appropriate Standard
Choose a reference standard compound with similar chemical properties to the analyte. This compound should be well-characterized, and its concentration should be known.
2. Prepare Calibration Curves
Prepare standard solutions of the reference compound and the analyte. Run these solutions under the same chromatographic conditions and generate calibration curves for each compound, plotting peak area (or height) versus concentration.
3. Measure Responses
Record the peak areas or heights of the analyte and the reference compound in the chromatogram. These responses will be used in the RRF calculation.
4. Calculate the RRF
Using the formula mentioned above, calculate the RRF by comparing the peak responses and concentrations of the analyte and the reference standard. Make sure the concentration units are consistent for accurate calculations.
5. Apply the RRF in Quantification
Once the RRF is determined, you can use it to calculate the concentration of the analyte in unknown samples based on its peak area and the peak area of the standard.
Example of RRF Calculation
Let’s assume you are analyzing a pharmaceutical sample containing a drug (analyte) and you are using caffeine as a reference standard. You prepare standard solutions of caffeine and the drug, run them through the HPLC, and get the following data:
| Compound | Concentration (mg/mL) | Peak Area |
|---|---|---|
| Caffeine (Standard) | 1.0 | 5000 |
| Drug (Analyte) | 0.5 | 3000 |
Now, calculate the RRF for the drug using the formula:
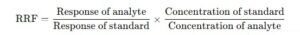
Substituting the values:
RRF=30005000×1.00.5=0.6\text{RRF} = \frac{3000}{5000} \times \frac{1.0}{0.5} = 0.6
The RRF for the drug is 0.6. You can now use this RRF to quantify the drug in future samples by comparing its response to the standard.
Factors Affecting RRF
Several factors can influence the RRF in HPLC, including:
-
Detector Sensitivity: Some detectors may respond differently to different compounds, especially for UV detection where the absorbance varies with wavelength.
-
Chemical Structure of the Compounds: Differences in chemical structure (such as molecular weight, polarity, or functional groups) can lead to varying detector responses.
-
Mobile Phase and Chromatographic Conditions: The composition of the mobile phase, flow rate, temperature, and column type can all impact the elution behavior of compounds, which in turn affects their detector responses.
-
Interfering Substances: Matrix effects or the presence of interfering substances in the sample can alter the detector’s response and affect the accuracy of the RRF.
Conclusion
The Relative Response Factor (RRF) plays a crucial role in ensuring the accuracy and reliability of HPLC quantification. By correcting for differences in detector response to various analytes, RRF allows for precise concentration determination, making it a valuable tool in analytical chemistry. Properly calculating and applying the RRF can significantly improve the quality and accuracy of HPLC results, especially when analyzing complex samples containing multiple compounds.
🎓 Discover one of the best Quality Assurance courses available — click below to explore the course that’s shaping future QA skills.
https://trcjw.on-app.in/app/oc/306166/trcjw

