Q12: Lifecycle Management
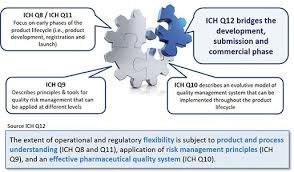
Lifecycle Management (LCM) in the pharmaceutical industry refers to the systematic approach of managing a product from its development phase through commercialization, post-approval changes, and discontinuation. It ensures continuous product quality, compliance, and supply throughout its lifecycle.
Key Elements of Lifecycle Management:
-
Development Phase:
-
Formulation design, analytical method development, and process optimization.
-
Establishing control strategies and specifications.
-
-
Technology Transfer:
-
Ensuring consistent product performance during scale-up or site transfer.
-
Documentation and validation of manufacturing and analytical processes.
-
-
Commercialization Phase:
-
Continuous process verification (CPV).
-
Routine manufacturing under validated conditions.
-
-
Post-Approval Changes:
-
Managing variations (CMC changes) per regulatory guidelines (e.g., ICH Q12).
-
Continuous improvement based on process data and quality metrics.
-
-
Discontinuation Phase:
-
Proper documentation, stability data management, and batch record archiving.
-
ICH Reference:
-
ICH Q12: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management — provides a framework for managing post-approval CMC changes efficiently and predictably.
Objective:
To maintain a state of control and ensure product quality, safety, and efficacy throughout the product lifecycle.

