
by Dr. Yashashwini Reddy | May 19, 2025
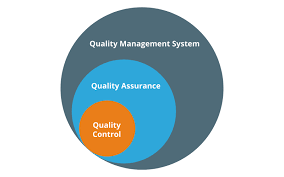
A Quality Management System (QMS) is a structured framework comprising processes, procedures, and responsibilities aimed at consistently delivering products or services that meet customer expectations and regulatory requirements. It serves as the backbone of an...

by Dr. Yashashwini Reddy | May 19, 2025
Gemba Walks, a cornerstone of Lean Management, are instrumental in enhancing operational efficiency and quality in pharmaceutical manufacturing. By observing processes directly on the production floor, organizations can identify inefficiencies, engage...

by Dr. Yashashwini Reddy | May 3, 2025
Enhancing Efficiency and Quality: Implementing Lean Manufacturing Principles in Pharmaceutical Manufacturing- The pharmaceutical industry faces increasing pressure to produce high-quality products efficiently, adhering to strict regulatory standards, while managing...
by Dr. Yashashwini Reddy | Apr 29, 2025
Supplier audits have a significant and multifaceted impact on quality assurance in the pharmaceutical sector. Here’s a detailed breakdown of their influence: 1. Ensuring Compliance with Regulatory Standards Supplier audits help ensure that raw material...

by Dr. Yashashwini Reddy | Apr 28, 2025
Introduction In the pharmaceutical industry, ensuring product quality, patient safety, and regulatory compliance is critical. When deviations, non-conformances, or process failures occur, it is essential to not only correct the immediate problem but also to identify...







