
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish a procedure for regular inspection of utilities (e.g., purified water, compressed air, HVAC system, steam, and gas supply) to ensure compliance with GMP requirements and operational efficiency. 2. Scope This procedure applies to all utility...

by Dr. Yashashwini Reddy | Sep 13, 2025
1. System Design & Validation Water system (Purified Water, WFI, Potable Water) designed to meet pharmacopeial requirements (USP/EP/IP/JP). System qualification performed (DQ, IQ, OQ, PQ) with proper documentation. Risk assessment performed for potential...

by Dr. Yashashwini Reddy | Sep 6, 2025
Case Studies: Troubleshooting Purified Water System Failures A Purified Water (PW) System must consistently supply water that meets pharmacopeial standards (USP, EP, IP, JP) for use in manufacturing, cleaning, and testing. Failures in the system can lead to OOS...
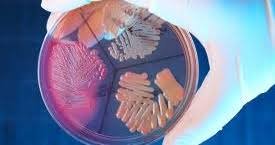
by Dr. Yashashwini Reddy | Aug 27, 2025
How to Remove Pathogens from Water Systems In pharmaceutical water systems (Purified Water, WFI, and water used in clean utilities), controlling and removing microbial pathogens is critical to meet GMP and pharmacopeial standards. 1. Source Control Use protected and...






