
by Dr. Yashashwini Reddy | Sep 8, 2025
Trends in GMP Violations in Pharmaceuticals Good Manufacturing Practice (GMP) violations remain one of the leading causes of FDA 483s, Warning Letters, and drug recalls. Recent inspection trends show recurring compliance gaps across global pharma companies: 1. Data...
by Dr. Yashashwini Reddy | Sep 2, 2025
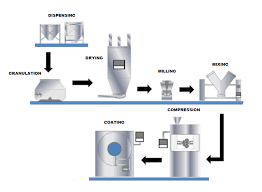
🔹 Tablet Manufacturing Process: An Overview Tablets are solid dosage forms containing one or more active pharmaceutical ingredients (APIs) with suitable excipients. The manufacturing process must ensure uniformity, stability, safety, and efficacy. 1. Pre-Formulation...

by Dr. Yashashwini Reddy | May 7, 2025
Standard Operating Procedure (SOP) 1. Purpose The purpose of this SOP is to outline the procedure for cleaning the tablet counter, ensuring that it operates efficiently, maintains product quality, and prevents contamination. Regular cleaning...

by Dr. Yashashwini Reddy | May 6, 2025
The tooling of Oral Solid Dosage Forms (OSDFs), especially tablets, plays a crucial role in ensuring the efficient and consistent manufacturing of high-quality pharmaceutical products. The term “tooling” refers to the specialized equipment and devices used...

by Dr. Yashashwini Reddy | May 6, 2025
A Checklist to Review Tablet Process Validation is essential for ensuring that tablet manufacturing processes are consistently producing products that meet predefined specifications and regulatory requirements. Process validation in pharmaceutical manufacturing is a...







