
by Dr. Yashashwini Reddy | Aug 28, 2025
1.0 Purpose To describe the procedure for performing the Bowie-Dick test to assess steam penetration and air removal efficiency in steam sterilizers (autoclaves). 2.0 Scope This SOP applies to all steam sterilizers (autoclaves) used in pharmaceutical manufacturing,...

by Dr. Yashashwini Reddy | Aug 28, 2025
1.0 Purpose To define the procedure for the preparation of disinfectant solutions used in pharmaceutical/biotechnology facilities for cleaning and sanitization purposes. 2.0 Scope This SOP applies to all personnel involved in the preparation, dilution, and labeling of...

by Dr. Yashashwini Reddy | Aug 27, 2025
Maintenance of Aseptic Conditions in Pharmaceutical Sterile Areas 1. Introduction Sterile areas in pharmaceutical manufacturing (e.g., aseptic filling, compounding, cleanrooms, isolators) require strict control to prevent contamination. Maintaining aseptic conditions...
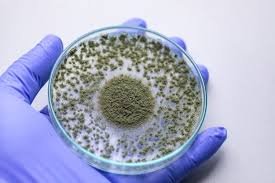
by Dr. Yashashwini Reddy | Aug 27, 2025
Fungus in Pharmaceutical Cleanrooms: Types, Origins, and Decontamination 1. Introduction Cleanrooms in pharmaceutical facilities are designed to maintain controlled levels of particles, microorganisms, and environmental parameters to ensure product sterility and...

by Dr. Yashashwini Reddy | Aug 27, 2025
Data Integrity in Microbial Analysis 1. Introduction In microbiology laboratories (especially in pharmaceutical QC/QA), data integrity ensures that all results of microbial testing are accurate, complete, consistent, and trustworthy throughout their lifecycle. Since...







