by Dr. Yashashwini Reddy | Sep 19, 2025
Water for Injection (WFI) in Pharmaceuticals Definition Water for Injection (WFI) is the highest purity water used in the pharmaceutical industry. It meets pharmacopeial standards (USP, EP, IP, JP) and is free from pyrogens, endotoxins, and microbial contamination....
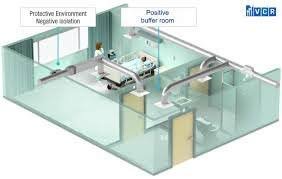
by Dr. Yashashwini Reddy | Sep 2, 2025
🔹 Buffer Area in Sterile Facility 1. Definition The Buffer Area (also called the cleanroom or critical area) is a Class 100 / ISO 5 / Grade A-B environment where aseptic processing, sterile filling, and direct product exposure operations are performed. It is...
by Dr. Yashashwini Reddy | Sep 2, 2025
📌 Restricted Access Barrier System (RABS) in Pharmaceuticals A Restricted Access Barrier System (RABS) is an advanced containment and protection technology used in pharmaceutical sterile manufacturing to reduce contamination risks. It provides a physical and...

by Dr. Yashashwini Reddy | Aug 27, 2025
Media Fill Test — Sterile API Manufacturing (Aseptic Process Simulation) 1. Objective To demonstrate that the aseptic API manufacturing process (including personnel, equipment, materials, and environment) can reliably produce a sterile product by simulating routine...

by Dr. Yashashwini Reddy | Aug 18, 2025
Types of Purified Water Systems in Pharmaceuticals Pharmaceutical industries require high-quality water for manufacturing, cleaning, and formulation. Different water systems are designed to meet specific pharmacopeial standards (USP, EP, IP, JP) depending on their...






