by Dr. Yashashwini Reddy | Sep 29, 2025
Advantages of Pharmaceutical Quality Audits Regulatory Compliance – Ensures adherence to GMP, FDA, EMA, WHO, and other regulatory requirements. Risk Identification – Detects potential risks, deviations, and non-compliance before they become critical issues. Continuous...

by Dr. Yashashwini Reddy | Sep 15, 2025
Annual Product Quality Review (APQR/APR/PQR) in Quality Improvements 1. What is APQR/APR/PQR? Annual Product Quality Review (APQR), also known as Annual Product Review (APR) or Product Quality Review (PQR), is a regulatory requirement under ICH Q7, EU GMP Chapter 1...

by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 Checklist for Internal Audit / Self-Inspection (Defects & Regulatory Compliance) 1. Documentation & Data Integrity ✅ Are records complete, contemporaneous, and accurate (ALCOA+ principles)? ✅ Any overwriting, missing data, or backdated entries? ✅ Are...

by Dr. Yashashwini Reddy | Sep 9, 2025
🏭 How FDA Inspections are Conducted in Manufacturing Facilities 1. Pre-Inspection Phase FDA identifies facilities to inspect based on: Risk-based selection (product type, compliance history, criticality, recalls, complaints). New drug approval or pre-approval...
by Dr. Yashashwini Reddy | Sep 9, 2025
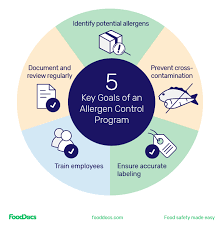
🧾 Allergen Control Plan for Pharmaceuticals 1. Risk Assessment Identify allergenic excipients (e.g., lactose, soy lecithin, egg-derived albumin, peanut oil, gluten, gelatin). Assess risk of cross-contamination during manufacturing, packaging, storage, and cleaning....





