by Dr. Yashashwini Reddy | Oct 8, 2025
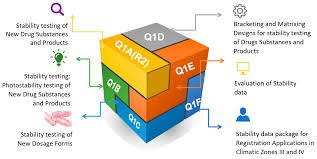
📘 ICH Q1: Stability Testing 🔹 Purpose The ICH Q1 series provides guidance on how to design stability studies to ensure that drug substances and drug products maintain their quality, safety, and efficacy throughout their shelf life under various environmental...

by Dr. Yashashwini Reddy | Aug 8, 2025
Strategies for Resolving Stability Issues in Drug Formulations Stability issues can arise due to chemical degradation, physical changes, or microbial contamination, leading to reduced efficacy, altered safety, or shortened shelf life. The resolution process involves...

by Dr. Yashashwini Reddy | Apr 28, 2025
Enhancing the stability of pharmaceutical formulations is a critical aspect of drug development and manufacturing. Stability ensures that the active ingredients in a drug maintain their potency, safety, and effectiveness throughout the product’s shelf life....

by Dr. Yashashwini Reddy | Apr 28, 2025
Understanding the Stability of Injectable Products Injectable products — such as vaccines, biologics, and sterile pharmaceuticals — must maintain strict stability to ensure their safety, efficacy, and quality throughout their shelf life. Stability refers to the...

by Dr. Yashashwini Reddy | Apr 19, 2025
1. What are stability studies and why are they important? Stability studies determine how the quality of a drug substance or product varies over time under the influence of environmental factors (e.g., temperature, humidity, light). They are essential for setting...







