
by Dr. Yashashwini Reddy | Sep 15, 2025
Internal Audit / Self-Inspection Checklist (Defects & Regulatory Compliance Focus) 1. Documentation & Records Are SOPs, policies, and work instructions up-to-date, approved, and controlled? Is there evidence of uncontrolled or obsolete documents in use? Are...

by Dr. Yashashwini Reddy | Sep 15, 2025
Self-Inspection and Quality Audits in Pharmaceuticals 1. Self-Inspection Definition: An internal examination carried out by the company itself to evaluate compliance with GMP, SOPs, and regulatory standards. Purpose: Detect deficiencies in the quality system. Verify...

by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 Checklist for Internal Audit / Self-Inspection (Defects & Regulatory Compliance) 1. Documentation & Data Integrity ✅ Are records complete, contemporaneous, and accurate (ALCOA+ principles)? ✅ Any overwriting, missing data, or backdated entries? ✅ Are...
by Dr. Yashashwini Reddy | Sep 9, 2025
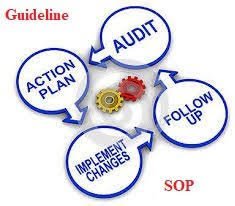
✅ Areas to Inspect During Self-Inspection in Pharmaceuticals Personnel & Training Verify staff qualifications, training records, and adherence to GMP practices. Premises & Facilities Check cleanliness, maintenance, pest control, HVAC systems, and environmental...
by Dr. Yashashwini Reddy | Aug 18, 2025
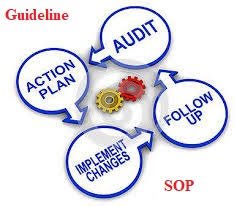
Self-Inspection and Its Implementation in Pharmaceuticals Definition:Self-inspection is a systematic, independent, and documented internal audit conducted by a pharmaceutical company to evaluate compliance with Good Manufacturing Practices (GMP), regulatory...







