
by Dr. Yashashwini Reddy | Sep 15, 2025
Critical Mistakes During Root Cause Investigation (RCI) Root Cause Investigation (RCI) is an essential step in pharmaceutical quality systems to identify, analyze, and eliminate the underlying causes of deviations, OOS, failures, or non-conformances. However, many...
by Dr. Yashashwini Reddy | Sep 10, 2025
🔴 Critical Mistakes in Root Cause Investigation (RCI): Jumping to Conclusions – Assuming the cause without evidence or proper investigation. Superficial Investigation – Stopping at symptoms instead of identifying the true underlying cause. Poor Documentation –...
by Dr. Yashashwini Reddy | Sep 9, 2025
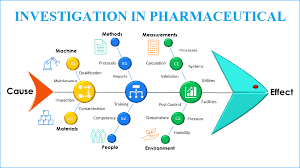
🐟 Fishbone Tool of Investigation in Pharmaceuticals 📌 What is It? A cause-and-effect diagram shaped like a fish skeleton. “Head” = problem statement (e.g., OOS result, contamination, deviation). “Bones” = major categories of potential causes. Helps investigation teams...
by Dr. Yashashwini Reddy | Aug 9, 2025
Common Causes of Low Quality in Pharmaceuticals Ensuring high-quality pharmaceuticals is crucial to patient safety, regulatory compliance, and brand reputation. Low-quality products can lead to therapeutic failure, adverse reactions, and recalls. The following are...
by Dr. Yashashwini Reddy | Aug 9, 2025
Non-conformance in Pharmaceuticals Definition:Non-conformance refers to any deviation from established standards, specifications, regulatory requirements, or approved procedures in the pharmaceutical manufacturing, testing, or distribution process. Examples of...







