by Dr. Yashashwini Reddy | Oct 9, 2025
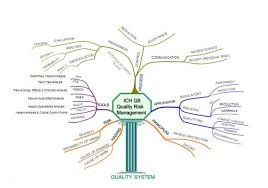
Quality Risk Management (QRM) is a systematic process for the assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. The purpose of QRM is to ensure that product quality, patient safety, and regulatory...
by Dr. Yashashwini Reddy | Aug 27, 2025
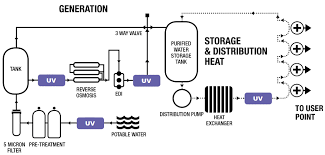
Risk Assessment for Purified Water System in Pharmaceuticals 1. Objective To identify, evaluate, and control risks associated with the design, operation, and maintenance of the Purified Water System (PWS), ensuring compliance with GMP, pharmacopeial standards, and...
by Dr. Yashashwini Reddy | Nov 5, 2024
Temperature Mapping in Pharmaceutical Storage Areas: Procedures and Best Practices Temperature mapping is a foundational process in pharmaceutical storage to ensure products are kept within prescribed temperature ranges. Proper mapping procedures, supported by...

by Srikanth | Sep 22, 2024
How is a risk assessment for computer systems carried out? A risk assessment is performed by identifying potential threats, analyzing their likelihood and impact, and implementing strategies to mitigate risks to an acceptable level. How do you ensure compliance with...
by Dr. Yashashwini Reddy | Jun 30, 2024
Computer system validation, if you are thinking about starting your career in CSV, then this article will give you an overview of what CSV is, and what is the role of CSV in the Pharma industry. CSV is a critical requirement of regulatory agencies. It aims to ensure...





