by Dr. Yashashwini Reddy | Oct 8, 2025
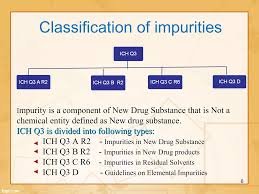
Elaboration:Impurities are unwanted chemicals that remain with the active pharmaceutical ingredient (API) or develop during formulation and storage. According to ICH Q3A (for new drug substances) and ICH Q3B (for new drug products), impurities must be identified,...
by Dr. Yashashwini Reddy | Sep 16, 2024
Residual solvents are volatile organic chemicals that remain in pharmaceutical products even after the completion of the manufacturing process. These solvents are often used in the production of active pharmaceutical ingredients (APIs), excipients, or the final drug...



