by Dr. Yashashwini Reddy | Aug 18, 2025
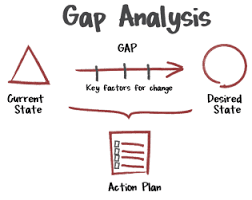
Gap Analysis for Regulatory Compliance Gap Analysis is a structured approach used in pharmaceutical and life sciences industries to identify the differences (“gaps”) between current practices and regulatory requirements or industry standards (e.g., FDA, EMA, ICH,...

by Dr. Yashashwini Reddy | Aug 18, 2025
Quality Inspection in Pharmaceuticals Quality inspection in pharmaceuticals is a critical step to ensure that drug products meet regulatory standards, patient safety requirements, and therapeutic efficacy. It involves systematic checks, tests, and verification across...
by Dr. Yashashwini Reddy | Aug 18, 2025
📌 Temperature and Humidity Validation/Mapping in Storage Area Overview:Temperature and humidity mapping is a critical activity in pharmaceutical warehouses, cold rooms, and storage areas to ensure that medicinal products are stored under controlled conditions as per...

by Dr. Yashashwini Reddy | Aug 18, 2025
Why Firms Must Avoid FDA 483 and Warning Letters? Pharmaceutical, biotechnology, and medical device firms must avoid FDA Form 483 observations and Warning Letters because they indicate serious compliance failures that can harm business operations, reputation, and...
by Dr. Yashashwini Reddy | Aug 18, 2025
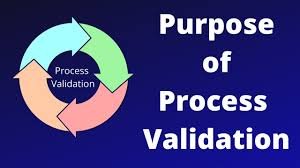
Purpose of Process Validation in Pharmaceuticals Process validation is a documented evidence-based approach that ensures a manufacturing process, when operated within established parameters, can consistently produce pharmaceutical products meeting predetermined...






