by Dr. Yashashwini Reddy | Sep 6, 2025
Planning and Procedure Followed During Regulatory Audits Regulatory audits (by USFDA, EMA, MHRA, WHO, or local authorities) are conducted to verify compliance with cGMP and regulatory standards. Effective preparation and systematic execution are crucial for success....
by Dr. Yashashwini Reddy | Sep 6, 2025
Regulatory Expectations from Cleaning Validation Cleaning validation is a documented evidence-based process to demonstrate that cleaning procedures effectively and consistently remove residues of products, cleaning agents, and contaminants to predetermined acceptable...

by Dr. Yashashwini Reddy | Sep 6, 2025
1.0 Purpose To lay down the procedure for the safe, secure, and documented transfer of finished goods from the production/packing area to the bonded store room under controlled conditions to ensure compliance with regulatory and company requirements. 2.0 Scope This...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To establish a uniform procedure for the operation, cleaning, and maintenance of the conveyor belt used in the packing section to ensure smooth functioning and compliance with cGMP requirements. 2.0 Scope This SOP applies to all conveyor belts used in the...
by Dr. Yashashwini Reddy | Sep 2, 2025
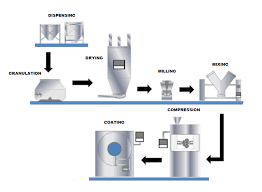
🔹 Tablet Manufacturing Process: An Overview Tablets are solid dosage forms containing one or more active pharmaceutical ingredients (APIs) with suitable excipients. The manufacturing process must ensure uniformity, stability, safety, and efficacy. 1. Pre-Formulation...





