by Dr. Yashashwini Reddy | Sep 9, 2025
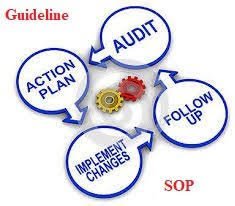
✅ Areas to Inspect During Self-Inspection in Pharmaceuticals Personnel & Training Verify staff qualifications, training records, and adherence to GMP practices. Premises & Facilities Check cleanliness, maintenance, pest control, HVAC systems, and environmental...
by Dr. Yashashwini Reddy | Sep 8, 2025
Planning and Execution of Internal Audits in Pharmaceuticals Internal audits (self-inspections) are a key part of a pharmaceutical Quality Management System (QMS). They ensure compliance with cGMP, regulatory guidelines, and company SOPs, while also driving continuous...

by Dr. Yashashwini Reddy | Sep 8, 2025
1. Be Inspection-Ready Always Keep documentation, equipment, and facilities in a state of compliance at all times. Conduct regular self-inspections and mock audits. Ensure all records are updated, accurate, and readily retrievable. 2. Train Employees on GMP and...
by Dr. Yashashwini Reddy | Sep 6, 2025
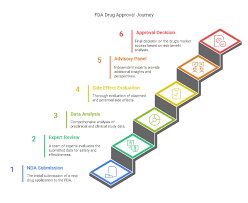
5 Steps of FDA Approvals The U.S. Food and Drug Administration (FDA) follows a structured process to ensure that drugs are safe, effective, and high-quality before they reach patients. 1. Preclinical Testing (Laboratory & Animal Studies) Conducted before human...

by Dr. Yashashwini Reddy | Sep 6, 2025
Requirements of FDA for Training in Pharmaceuticals Training in the pharmaceutical industry is a critical requirement under cGMP regulations (21 CFR Parts 210 & 211). The FDA expects companies to have a structured, documented training system that ensures employees...






