by Dr. Yashashwini Reddy | Sep 23, 2025
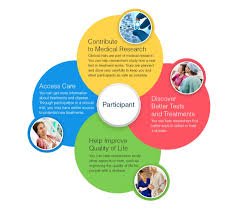
🔹 For Patients Access to New Treatments Early Patients may gain access to investigational drugs, biologics, or devices before they’re widely available. This can be especially important when current treatments are limited or ineffective. Closer Medical Monitoring...
by Dr. Yashashwini Reddy | Sep 20, 2025
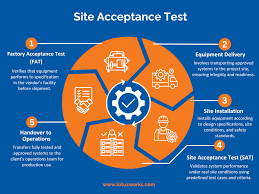
1. Objective To ensure that equipment, system, or instrument is installed correctly at the user site and performs as per the predefined specifications, operational requirements, and approved protocols. 2. Scope Applicable to all new equipment, utilities, systems, and...

by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish a documented procedure to verify that the equipment/system has been properly installed according to manufacturer’s specifications, approved drawings, and regulatory requirements. 2. Scope This SOP applies to all new equipment, instruments,...

by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To describe the procedure for performing and documenting Operational Qualification (OQ) of equipment, instruments, and systems to ensure they operate according to defined specifications and intended performance. 2. Scope This procedure applies to all...

by Dr. Yashashwini Reddy | Sep 19, 2025
Periodic Review and Requalification 1. Periodic Review Definition:A systematic review of facilities, utilities, equipment, and computerized systems at defined intervals to ensure they remain in a state of control and continue to comply with regulatory requirements....







