
by Dr. Yashashwini Reddy | Sep 29, 2025
Aspect Audit Inspection Definition A systematic, independent review to assess compliance with internal or external standards (e.g., GMP). A formal review by a regulatory authority (e.g., FDA, EMA) to ensure compliance with laws and regulations. Conducted By Internal...

by Dr. Yashashwini Reddy | Sep 23, 2025
Case Study: Supply Chain Disruption – API Shortage Background A U.S.-based pharmaceutical company manufacturing essential cardiovascular and anti-diabetic medicines faced a sudden API (Active Pharmaceutical Ingredient) shortage in 2024. The primary API supplier was...
by Dr. Yashashwini Reddy | Sep 23, 2025
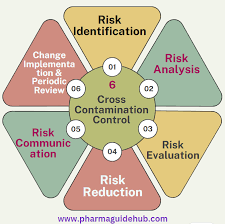
🧪 Case Study: Cross-Contamination in a Multi-Product Pharmaceutical Facility 📌 Background A European pharmaceutical manufacturer operated a multi-product solid oral dosage plant. During a routine EMA inspection, regulatory authorities found traces of a potent API...

by Dr. Yashashwini Reddy | Sep 23, 2025
📝 Case Study: OOS Investigation – Tablet Dissolution 📍 Background A marketed immediate-release tablet showed dissolution failure during routine quality control testing. Specification: NLT (Not Less Than) 80% drug release in 30 minutes. Observed result: 60–65% release...
by Dr. Yashashwini Reddy | Sep 23, 2025
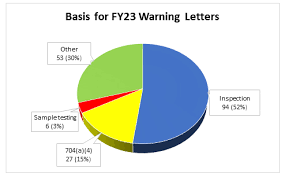
📌 Case Study: Data Integrity Failures – FDA Warning Letters 1. Background In recent years, several pharmaceutical companies (especially in India and China) have faced FDA Warning Letters and import alerts because of data integrity violations. These cases highlight the...







