
by Dr. Yashashwini Reddy | Jun 24, 2025
🗂️ GVP Module II: Pharmacovigilance System Master File (PSMF) 🔹 Purpose GVP Module II provides guidance on the structure, content, maintenance, and location of the Pharmacovigilance System Master File (PSMF), a key document required for all Marketing Authorization...

by Dr. Yashashwini Reddy | Jun 9, 2025
Purpose of Process Validation in Pharmaceuticals Process validation is a critical activity in pharmaceutical manufacturing that ensures a process consistently produces a product meeting its predetermined quality attributes. The purpose of process validation is to...
by Dr. Yashashwini Reddy | Jun 9, 2025
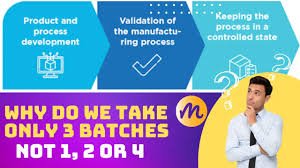
Three Consecutive Batches for Validation in Pharmaceuticals Definition: In pharmaceutical manufacturing, validation is the documented evidence that a process consistently produces a product meeting its predetermined quality specifications. One of the critical...

by Dr. Yashashwini Reddy | Jun 9, 2025
🔹 What is Contamination in Pharma Manufacturing? Contamination refers to the unintended presence of chemical, microbial, particulate, or cross-substance materials in a product or manufacturing environment. It can affect product safety, efficacy, and regulatory...

by Dr. Yashashwini Reddy | May 19, 2025
The U.S. Food and Drug Administration (FDA) has issued multiple warning letters to pharmaceutical and medical device manufacturers for deficiencies in cleaning validation processes. These letters highlight the critical importance of robust cleaning procedures to...







