
by Dr. Yashashwini Reddy | Aug 9, 2025
Pharmaceutical Compliance and Product Quality 1. Pharmaceutical Compliance Definition:Adherence to all applicable laws, regulations, guidelines, and internal SOPs that govern pharmaceutical manufacturing, testing, storage, and distribution. Key Compliance Areas: GMP...
by Dr. Yashashwini Reddy | Aug 9, 2025
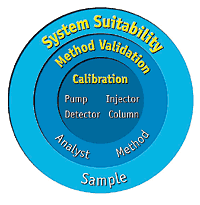
System Suitability in HPLC Analysis What is System Suitability? System Suitability Testing (SST) is a set of analytical checks performed before and during analysis to ensure that the HPLC system and method are capable of producing accurate, precise, and reproducible...
by Dr. Yashashwini Reddy | Aug 9, 2025
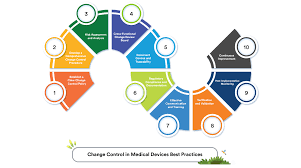
4 Steps to Effective Change Control in Pharmaceuticals 1. Initiation & Documentation Identify the need for change (equipment, process, material, method, etc.). Document the proposed change in a Change Control Form with details like scope, reason, impact area, and...

by Dr. Yashashwini Reddy | Jun 26, 2025
✅ Pharmacovigilance Audits and Inspections 🔍 1. Pharmacovigilance Audits 📘 Definition: A systematic, independent, and documented process to evaluate the pharmacovigilance (PV) system, its quality system, and compliance with regulatory requirements. 📌...

by Dr. Yashashwini Reddy | Jun 25, 2025
✅ GVP Module VI – Addendum I: Duplicate Management of Suspected Adverse Reaction Reports 🔹 Purpose This Addendum to GVP Module VI provides guidance on how Marketing Authorisation Holders (MAHs) and National Competent Authorities (NCAs) should manage duplicate...







