
by Dr. Yashashwini Reddy | Aug 10, 2025
QC Audit Checklist 1. General Documentation & Records SOPs for all QC activities (latest approved versions, controlled copies). Records of training, competency assessments, and analyst qualification. QC logbooks (instrument usage, calibration, maintenance). Change...
by Dr. Yashashwini Reddy | Aug 10, 2025
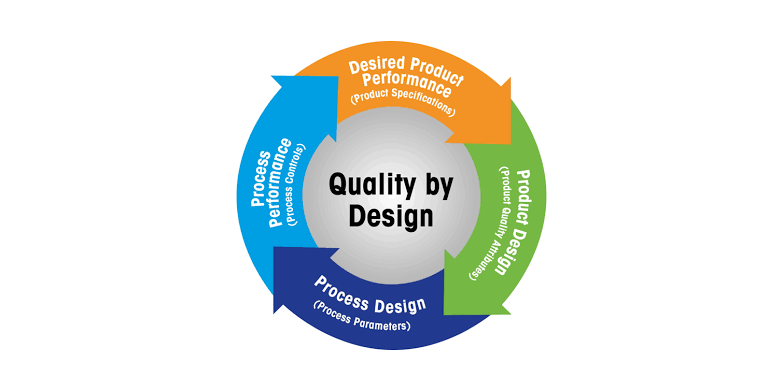
Quality by Design (QbD) in Pharmaceuticals Definition:Quality by Design (QbD) is a systematic, risk-based, proactive approach to pharmaceutical development that emphasizes designing and building quality into the product from the very beginning, rather than relying...

by Dr. Yashashwini Reddy | Aug 10, 2025
Requirements and Implementation of Continuous Training in Pharmaceuticals Continuous training in the pharmaceutical industry is a regulatory, quality, and operational necessity to ensure that employees remain competent, compliant, and up-to-date with evolving...

by Dr. Yashashwini Reddy | Aug 10, 2025
Handling of Out of Calibration (OOC) Instruments and Equipment Definition Out of Calibration (OOC) refers to an instrument or equipment that, during calibration verification, fails to meet the specified acceptance criteria or shows results outside its defined...
by Dr. Yashashwini Reddy | Aug 9, 2025
Common Causes of Low Quality in Pharmaceuticals Ensuring high-quality pharmaceuticals is crucial to patient safety, regulatory compliance, and brand reputation. Low-quality products can lead to therapeutic failure, adverse reactions, and recalls. The following are...







