by Dr. Yashashwini Reddy | Oct 8, 2025
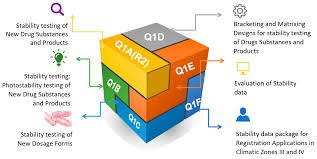
📘 ICH Q1: Stability Testing 🔹 Purpose The ICH Q1 series provides guidance on how to design stability studies to ensure that drug substances and drug products maintain their quality, safety, and efficacy throughout their shelf life under various environmental...

by Dr. Yashashwini Reddy | Sep 29, 2025
📋 Types of Audits in the Pharmaceutical Industry 1. Internal Audit (Self-Inspection) Purpose: To assess compliance with internal SOPs and GMP requirements. Conducted By: Company’s own QA or compliance team. Frequency: Regularly scheduled (e.g., annually or quarterly)....

by Dr. Yashashwini Reddy | Sep 18, 2025
📌 Importance of Differential Pressure in Pharmaceuticals Prevents Cross-Contamination Differential pressure ensures controlled airflow between cleanrooms of different classifications. Positive pressure in cleaner areas prevents entry of dust, microbes, and...
by Dr. Yashashwini Reddy | Sep 6, 2025
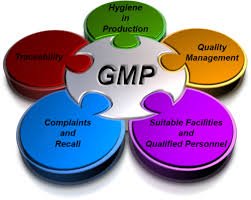
Current Good Manufacturing Practices (cGMP) in Pharmaceutical Industries Current Good Manufacturing Practices (cGMP) are the regulatory standards enforced by agencies like the USFDA, WHO, and EMA to ensure that pharmaceutical products are consistently produced and...

by Dr. Yashashwini Reddy | Jun 30, 2025
📘 Regulations: Good Clinical Practice (GCP) and Clinical Trials Good Clinical Practice (GCP) is an international ethical and scientific standard for designing, conducting, monitoring, recording, auditing, analyzing, and reporting clinical trials that involve human...







