
by Dr. Yashashwini Reddy | Aug 12, 2025
Change Control in Pharmaceuticals Definition:Change control is a formal GMP-compliant system used to manage and document any change in processes, equipment, materials, methods, documents, or facilities to ensure that product quality, safety, and regulatory...
by Dr. Yashashwini Reddy | Aug 12, 2025
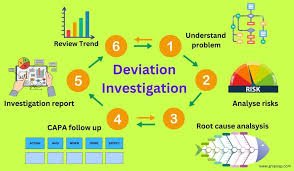
Deviation Control in Pharmaceuticals Definition:A deviation is any departure from approved instructions, standard operating procedures (SOPs), specifications, or established Good Manufacturing Practice (GMP) requirements during manufacturing, testing, packaging,...
by Dr. Yashashwini Reddy | Aug 9, 2025
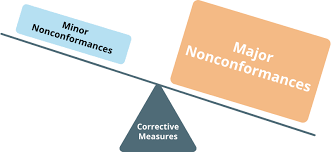
Non-conformance in Pharmaceuticals Definition:Non-conformance refers to any deviation from established standards, specifications, regulatory requirements, or approved procedures in the pharmaceutical manufacturing, testing, or distribution process. Examples of...

by Dr. Yashashwini Reddy | May 19, 2025
A Quality Management System (QMS) is a structured framework comprising processes, procedures, and responsibilities aimed at consistently delivering products or services that meet customer expectations and regulatory requirements. It serves as the backbone of an...
by Dr. Yashashwini Reddy | Apr 29, 2025
Non-conformance in the pharmaceutical industry refers to deviations from established standards, regulations, or specifications in the production, packaging, labeling, or testing of pharmaceutical products. These deviations can lead to serious consequences, including...






