
by Dr. Yashashwini Reddy | Sep 15, 2025
📘 Guidelines for Preparation of Site Master File (SMF) The Site Master File (SMF) is a regulatory document that provides detailed information about a pharmaceutical manufacturing site, operations, and quality management system. It is required by WHO, EU GMP (Annex...

by Dr. Yashashwini Reddy | Aug 12, 2025
Quality Management System (QMS) 1. Definition A Quality Management System (QMS) is a structured framework of policies, procedures, processes, and resources that ensures pharmaceutical products consistently meet quality, safety, efficacy, and regulatory requirements.It...
by Dr. Yashashwini Reddy | Aug 12, 2025
Aspect Process Validation Product Validation Definition Documented evidence that a manufacturing process, operated within established parameters, can consistently produce a product meeting its predetermined quality attributes. Documented evidence that a specific...
by Dr. Yashashwini Reddy | Aug 12, 2025
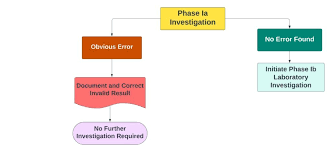
Investigation of OOS Results in Analytical Testing 1. Definition An Out of Specification (OOS) result occurs when an analytical test result for a product, raw material, or intermediate falls outside the approved acceptance criteria or specifications set by regulatory...

by Dr. Yashashwini Reddy | Aug 12, 2025
CAPA Documentation: Common Mistakes to Avoid Definition:Corrective and Preventive Actions (CAPA) are processes used in pharmaceutical quality systems to investigate, address, and prevent the recurrence of deviations, non-conformances, or quality issues. Proper...






