by Dr. Yashashwini Reddy | Oct 9, 2025
A Pharmaceutical Quality System (PQS) is a comprehensive framework that ensures the consistent quality, safety, and efficacy of pharmaceutical products throughout their life cycle — from development to discontinuation. It aligns with ICH Q10, which complements ICH Q8...

by Dr. Yashashwini Reddy | Sep 26, 2025
📌 Case Study: Sandoz Canada (2012) – Supply Shortages due to GMP Non-Compliance Background Company: Sandoz Canada, a division of Novartis Year: 2012 Issue: Nationwide shortage of injectable drugs in Canada and USA Cause: Non-compliance with Good Manufacturing...

by Dr. Yashashwini Reddy | Aug 9, 2025
Out of Specification (OOS) Investigation in Pharmaceuticals Definition OOS results are test results that fall outside the pre-established acceptance criteria defined in specifications, procedures, or regulatory filings.They can occur in raw materials, in-process...
by Dr. Yashashwini Reddy | Aug 9, 2025
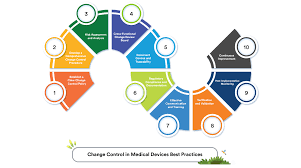
4 Steps to Effective Change Control in Pharmaceuticals 1. Initiation & Documentation Identify the need for change (equipment, process, material, method, etc.). Document the proposed change in a Change Control Form with details like scope, reason, impact area, and...

by Dr. Yashashwini Reddy | Jun 9, 2025
Definition: Self-inspection is a critical component of the pharmaceutical Quality Management System (QMS). It refers to the internal evaluation of all aspects of Good Manufacturing Practices (GMP) within a pharmaceutical facility. The goal is to ensure compliance with...







