
by Dr. Yashashwini Reddy | Sep 8, 2025
Difference Between FDA Form 483 and Warning Letter 1. FDA Form 483 (Inspectional Observations) Issued when: FDA inspectors observe potential violations of the Food, Drug, and Cosmetic Act (FD&C Act) during an inspection. Purpose: To notify company management of...

by Dr. Yashashwini Reddy | Sep 8, 2025
Drug recalls can harm patients, damage reputation, and lead to regulatory penalties. To minimize risks, pharmaceutical companies should adopt a proactive quality and compliance strategy. 1. Strong Quality Management System (QMS) Implement robust SOPs across...
by Dr. Yashashwini Reddy | Sep 5, 2025
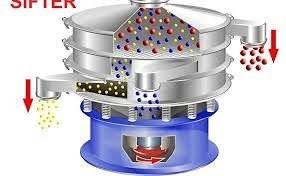
1.0 Purpose To define the procedure for operation, cleaning, and maintenance of the Vibro Sifter used in production to ensure efficient sieving of raw materials and granules as per cGMP requirements. 2.0 Scope This SOP applies to the Vibro Sifter installed in the...
by Dr. Yashashwini Reddy | Sep 3, 2025
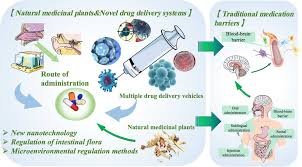
Novel Drug Delivery Systems (NDDS) A Novel Drug Delivery System is a modern approach of formulating and delivering drugs to improve efficacy, safety, patient compliance, and targeted delivery compared to conventional dosage forms (like tablets, capsules, or...

by Dr. Yashashwini Reddy | Sep 3, 2025
Ultrasonic Cleaning for Equipment and Tooling in Pharmaceuticals Ultrasonic cleaning is a modern cleaning method widely used in pharmaceutical manufacturing for equipment, tools, and small machine parts. It ensures thorough, consistent, and validated cleaning, which...







