
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish a procedure for regular inspection of utilities (e.g., purified water, compressed air, HVAC system, steam, and gas supply) to ensure compliance with GMP requirements and operational efficiency. 2. Scope This procedure applies to all utility...

by Dr. Yashashwini Reddy | Sep 15, 2025
Analytical Balances Drift and Its Importance 1. What is Analytical Balance Drift? Analytical balance drift refers to the gradual and unintentional change in the displayed weight reading of a balance over time, even without adding or removing material. This drift can...

by Dr. Yashashwini Reddy | Sep 15, 2025
Critical Mistakes During Root Cause Investigation (RCI) Root Cause Investigation (RCI) is an essential step in pharmaceutical quality systems to identify, analyze, and eliminate the underlying causes of deviations, OOS, failures, or non-conformances. However, many...
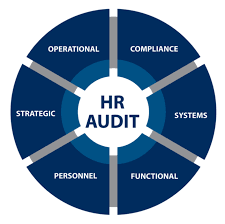
by Dr. Yashashwini Reddy | Sep 13, 2025
HR Audit Checklist 1. Recruitment & Selection Existence of recruitment policies and SOPs. Proper manpower requisition approvals before hiring. Background verification and reference checks of employees. Job descriptions and competency requirements documented....

by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 Checklist for Internal Audit / Self-Inspection (Defects & Regulatory Compliance) 1. Documentation & Data Integrity ✅ Are records complete, contemporaneous, and accurate (ALCOA+ principles)? ✅ Any overwriting, missing data, or backdated entries? ✅ Are...







