by Dr. Yashashwini Reddy | Aug 10, 2025
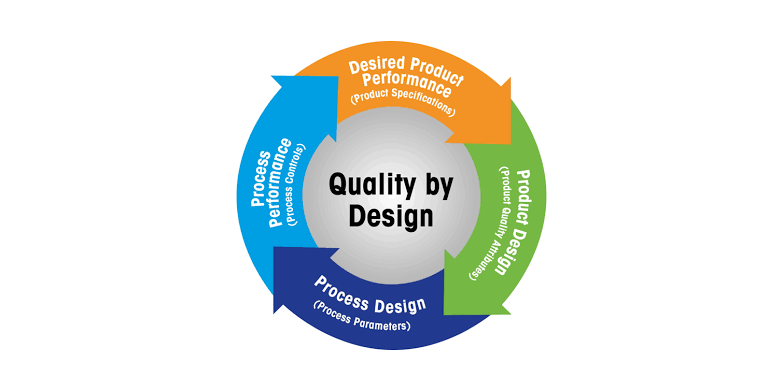
Quality by Design (QbD) in Pharmaceuticals Definition:Quality by Design (QbD) is a systematic, risk-based, proactive approach to pharmaceutical development that emphasizes designing and building quality into the product from the very beginning, rather than relying...

by Dr. Yashashwini Reddy | May 3, 2025
Improving pharmaceutical productivity and product quality is essential to meet the growing global demand for effective and safe medications. This can be achieved through a combination of strategies that address various aspects of pharmaceutical manufacturing, from raw...

by Dr. Yashashwini Reddy | May 1, 2025
Quality by Design (QbD) in Pharmaceuticals: A Detailed Explanation Quality by Design (QbD) is a systematic approach to pharmaceutical development that aims to ensure the quality of a drug product through the design and control of the manufacturing process. This...
by Dr. Yashashwini Reddy | Nov 26, 2024
Top Interview Questions for Experienced Candidates in Formulation R&D (Oral Solid Dosage) 1. Can you explain the pre-formulation studies conducted before developing an OSD? Answer: Pre-formulation studies involve evaluating the physicochemical properties of a drug...
by Dr. Yashashwini Reddy | Nov 25, 2024
Drug-Excipient Compatibility Study: Ensuring Safe and Effective Medicines Introduction In pharmaceutical development, ensuring that a drug remains stable and effective is essential. One important step in this process is the drug-excipient compatibility study. This...





