
by Dr. Yashashwini Reddy | Oct 9, 2025
Continuous Manufacturing (CM) is an advanced pharmaceutical production approach where input materials are continuously fed into the system and the processed output (finished product) is continuously removed. Unlike traditional batch manufacturing, which occurs in...
by Dr. Yashashwini Reddy | Oct 9, 2025
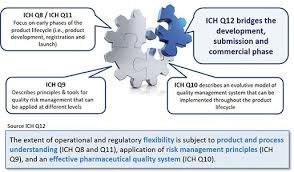
Lifecycle Management (LCM) in the pharmaceutical industry refers to the systematic approach of managing a product from its development phase through commercialization, post-approval changes, and discontinuation. It ensures continuous product quality, compliance, and...

by Dr. Yashashwini Reddy | Oct 9, 2025
Elaboration:The development and manufacture of drug substances (Active Pharmaceutical Ingredients – API) encompass all stages from the initial synthesis or isolation of the active compound to its large-scale commercial production under Good Manufacturing Practice...
by Dr. Yashashwini Reddy | Oct 9, 2025
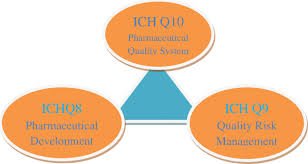
Pharmaceutical development, as described in ICH Q8 (R2), is the process of designing a quality product and its manufacturing process to consistently deliver the intended performance. The goal is to understand how formulation and process variables influence product...

by Dr. Yashashwini Reddy | Oct 8, 2025
🧭 Quality Guidelines – Overview Quality Guidelines are internationally harmonized standards developed mainly by the International Council for Harmonisation (ICH) and other regulatory authorities (like FDA, EMA, WHO, CDSCO).They help ensure that pharmaceutical products...







