
by Dr. Yashashwini Reddy | May 8, 2025
STANDARD OPERATING PROCEDURE (SOP) 1.0 OBJECTIVE To establish a standard procedure for cleaning the capsule filling line to ensure product quality, prevent cross-contamination, and comply with regulatory requirements. 2.0 SCOPE This SOP...
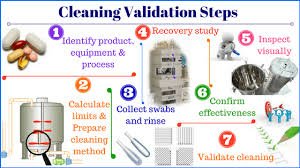
by Dr. Yashashwini Reddy | May 8, 2025
STANDARD OPERATING PROCEDURE (SOP) 1.0 OBJECTIVE To establish a standard procedure for the cleaning of equipment and accessories used in the production area to ensure cleanliness, prevent cross-contamination, and maintain product quality....

by Dr. Yashashwini Reddy | May 7, 2025
Standard Operating Procedure (SOP) 1. Objective To establish standardized procedures for reprocessing and reworking pharmaceutical products to ensure that all batches meet predefined specifications and maintain product quality. 2. Scope...

by Dr. Yashashwini Reddy | May 7, 2025
SOP for Failure Investigation in Pharmaceutical Manufacturing Here’s an overview of the Standard Operating Procedure (SOP) for Failure Investigation in pharmaceutical manufacturing, focusing on identifying root causes, implementing corrective and preventive...

by Dr. Yashashwini Reddy | May 7, 2025
Standard Operating Procedure (SOP) 1. Objective To establish a standardized method for the safe and efficient operation of the 20-station single rotary tablet compression machine, ensuring consistent tablet quality and compliance with Good...







