
by Dr. Yashashwini Reddy | Aug 12, 2025
How to Review GMP Documents Like a Pro 1. Understand the Purpose Identify what the document is for (e.g., SOP, batch record, protocol, report). Know the applicable GMP guidelines (US FDA, EMA, WHO, ICH). 2. Check for Compliance Ensure the document aligns with: Current...

by Dr. Yashashwini Reddy | Aug 12, 2025
Change Control in Pharmaceuticals Definition:Change control is a formal GMP-compliant system used to manage and document any change in processes, equipment, materials, methods, documents, or facilities to ensure that product quality, safety, and regulatory...
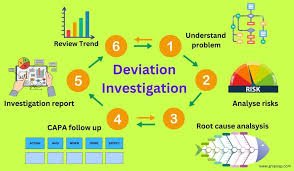
by Dr. Yashashwini Reddy | Aug 12, 2025
Deviation Control in Pharmaceuticals Definition:A deviation is any departure from approved instructions, standard operating procedures (SOPs), specifications, or established Good Manufacturing Practice (GMP) requirements during manufacturing, testing, packaging,...

by Dr. Yashashwini Reddy | Aug 11, 2025
Calibration of Viscometer 1. Introduction A viscometer is used to measure the viscosity of liquids, which is a critical quality attribute in pharmaceuticals, especially for syrups, suspensions, ointments, creams, and injectable formulations.Calibration ensures the...

by Dr. Yashashwini Reddy | Jun 9, 2025
What is a Validation Master Plan (VMP)? A Validation Master Plan is a high-level document that outlines the overall strategy, scope, approach, and responsibilities for validation activities within a pharmaceutical facility. It serves as a roadmap to ensure all...







