by Dr. Yashashwini Reddy | Aug 12, 2025
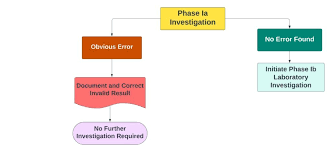
Investigation of OOS Results in Analytical Testing 1. Definition An Out of Specification (OOS) result occurs when an analytical test result for a product, raw material, or intermediate falls outside the approved acceptance criteria or specifications set by regulatory...
by Dr. Yashashwini Reddy | Aug 12, 2025
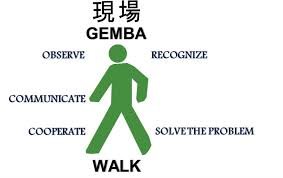
Gemba Walks and Their Implementation in Pharmaceuticals 1. What is a Gemba Walk? Gemba is a Japanese term meaning “the real place”—where work actually happens. In lean manufacturing and quality management, a Gemba Walk is when leaders, managers, or QA personnel go to...

by Dr. Yashashwini Reddy | Aug 12, 2025
CAPA Documentation: Common Mistakes to Avoid Definition:Corrective and Preventive Actions (CAPA) are processes used in pharmaceutical quality systems to investigate, address, and prevent the recurrence of deviations, non-conformances, or quality issues. Proper...

by Dr. Yashashwini Reddy | Aug 12, 2025
How to Submit a DMF (Drug Master File) A Drug Master File (DMF) is a confidential document submitted to the U.S. FDA that contains detailed information about the manufacturing, processing, packaging, and storage of drug substances, intermediates, or components.It is...

by Dr. Yashashwini Reddy | Aug 12, 2025
Understanding the US FDA Drug Approval Process The U.S. Food and Drug Administration (FDA) is responsible for ensuring that drugs marketed in the United States are safe, effective, and of high quality. The process is rigorous and involves multiple stages to verify...







