
by Dr. Yashashwini Reddy | Aug 18, 2025
Validation of Compressed Air in Pharmaceuticals Compressed air is widely used in pharmaceutical manufacturing for processes like cleaning, drying, aeration, packaging, and equipment operation. Since compressed air may directly or indirectly come in contact with...

by Dr. Yashashwini Reddy | Aug 18, 2025
Contamination Control Strategies for Manufacturing Area In pharmaceutical manufacturing, contamination control is critical to ensure product quality, patient safety, and regulatory compliance. Contamination can occur in the form of particulate, microbial, chemical, or...
by Dr. Yashashwini Reddy | Aug 18, 2025
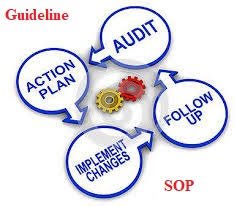
Self-Inspection and Its Implementation in Pharmaceuticals Definition:Self-inspection is a systematic, independent, and documented internal audit conducted by a pharmaceutical company to evaluate compliance with Good Manufacturing Practices (GMP), regulatory...

by Dr. Yashashwini Reddy | Aug 18, 2025
Site Acceptance Test (SAT) The Site Acceptance Test (SAT) is the final phase of equipment/system qualification, conducted at the customer’s site after installation and commissioning. It verifies that the system, machine, or equipment functions correctly in the actual...
by Dr. Yashashwini Reddy | Aug 18, 2025
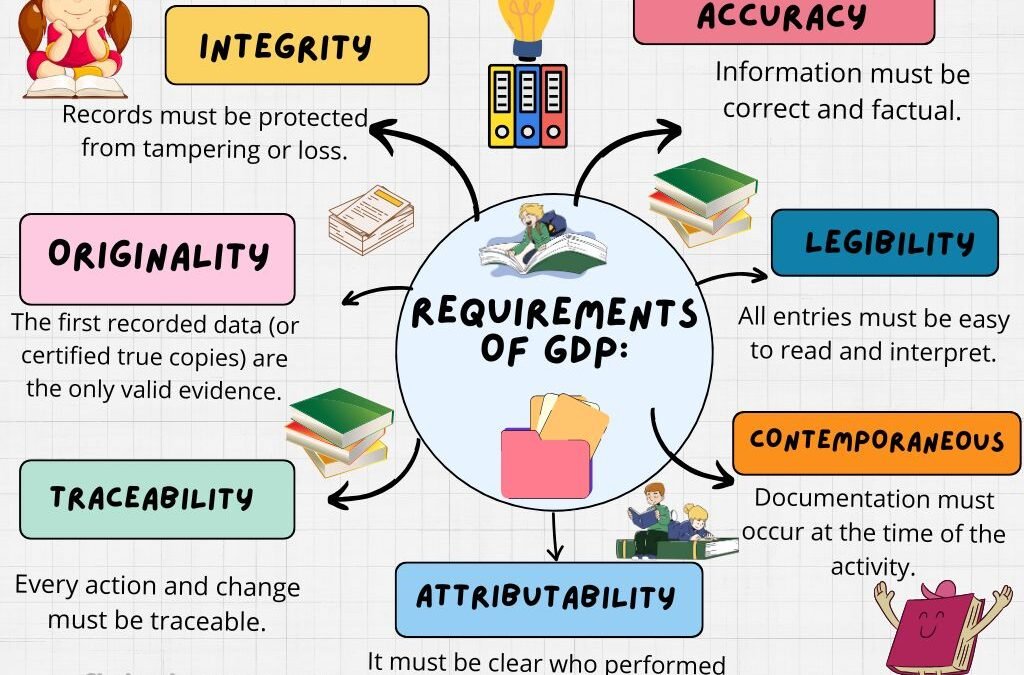
Requirements for Good Documentation Practice (GDP) Legibility All entries should be clear, readable, and permanent (no pencil or erasable ink). Accuracy Data should reflect the actual observation, measurement, or action without manipulation. Contemporaneous Recording...







