
by Dr. Yashashwini Reddy | Aug 18, 2025
Possible Causes of OOS Results 1. Laboratory Errors Improper sample preparation or dilution Incorrect weighing or pipetting Instrument malfunction or improper calibration Wrong method execution or deviation from SOP Calculation or transcription errors 2....

by Dr. Yashashwini Reddy | Aug 18, 2025
FDA Requirements for Training in Pharmaceuticals The U.S. Food and Drug Administration (FDA), under 21 CFR Part 211 (cGMP for Finished Pharmaceuticals) and ICH Q10 Pharmaceutical Quality System, outlines clear expectations for employee training. 1. Training Program...

by Dr. Yashashwini Reddy | Aug 18, 2025
✅ Tips to Develop Equipment Cleaning Procedure Risk Assessment First Identify the potential for cross-contamination, product carryover, and microbial risks. Classify equipment as product-contact or non-product-contact. Define Cleaning Scope Clearly specify which...
by Dr. Yashashwini Reddy | Aug 18, 2025
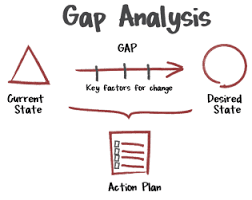
Gap Analysis for Regulatory Compliance Gap Analysis is a structured approach used in pharmaceutical and life sciences industries to identify the differences (“gaps”) between current practices and regulatory requirements or industry standards (e.g., FDA, EMA, ICH,...

by Dr. Yashashwini Reddy | Aug 18, 2025
Cleaning Validation of Manufacturing Equipment Cleaning validation is a documented process that ensures manufacturing equipment in the pharmaceutical industry is consistently cleaned to predetermined limits, preventing contamination and cross-contamination between...







