
by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To describe the procedure for the operation, cleaning, and maintenance of the Multimill to ensure consistent performance and compliance with cGMP requirements. 2.0 Scope This SOP applies to the Multimill installed in the production area of [Company Name],...

by Dr. Yashashwini Reddy | Sep 3, 2025
Human Error: Some Fresh Approaches to Consider Human errors are often seen as a root cause in pharmaceutical and other regulated industries, but simply blaming individuals is not enough.Fresh approaches focus on: ✅ System Design – Designing error-proof processes...
by Dr. Yashashwini Reddy | Sep 2, 2025
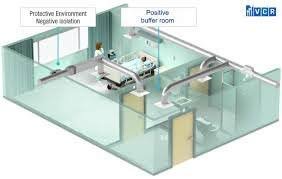
🔹 Buffer Area in Sterile Facility 1. Definition The Buffer Area (also called the cleanroom or critical area) is a Class 100 / ISO 5 / Grade A-B environment where aseptic processing, sterile filling, and direct product exposure operations are performed. It is...

by Dr. Yashashwini Reddy | Sep 2, 2025
🔹 1. Process Optimization Lean Manufacturing (Lean Pharma): Eliminate non-value-added steps, reduce waiting times, optimize material flow. Six Sigma & QbD (Quality by Design): Use statistical tools (DoE, risk assessment) to identify Critical Process Parameters...

by Dr. Yashashwini Reddy | Sep 2, 2025
🔹 Types of Stainless Steel in Pharmaceuticals 1. SS 304 / 304L (AISI 304) Composition: 18% Cr, 8% Ni 304L = low carbon version → better weldability, less sensitization Applications: Non-contact parts (frames, supports, furniture, piping for non-critical utilities)...







