by Dr. Yashashwini Reddy | Sep 23, 2025
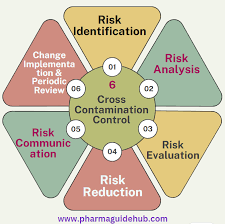
🧪 Case Study: Cross-Contamination in a Multi-Product Pharmaceutical Facility 📌 Background A European pharmaceutical manufacturer operated a multi-product solid oral dosage plant. During a routine EMA inspection, regulatory authorities found traces of a potent API...
by Dr. Yashashwini Reddy | Sep 23, 2025
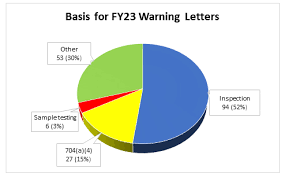
📌 Case Study: Data Integrity Failures – FDA Warning Letters 1. Background In recent years, several pharmaceutical companies (especially in India and China) have faced FDA Warning Letters and import alerts because of data integrity violations. These cases highlight the...

by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish a procedure for regular inspection of utilities (e.g., purified water, compressed air, HVAC system, steam, and gas supply) to ensure compliance with GMP requirements and operational efficiency. 2. Scope This procedure applies to all utility...

by Dr. Yashashwini Reddy | Sep 20, 2025
1. Premises and Equipment Use dedicated and clean facilities for production, storage, and filling of gases. Ensure gas cylinders, pipelines, valves, regulators, and manifolds are properly maintained and validated. Prevent cross-contamination between medical and...
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish guidelines for maintaining personal hygiene of all personnel to ensure product safety, prevent contamination, and maintain a clean working environment. 2. Scope This SOP applies to all employees, contract workers, visitors, and service...







