by Dr. Yashashwini Reddy | Sep 8, 2025
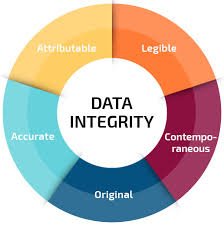
Data Integrity – A Major Problem in Pharmaceuticals Data integrity has become one of the most critical issues in the pharmaceutical industry, directly impacting patient safety, product quality, and regulatory trust. Regulatory agencies like the FDA, EMA, and MHRA...

by Dr. Yashashwini Reddy | Sep 8, 2025
Trends in GMP Violations in Pharmaceuticals Good Manufacturing Practice (GMP) violations remain one of the leading causes of FDA 483s, Warning Letters, and drug recalls. Recent inspection trends show recurring compliance gaps across global pharma companies: 1. Data...

by Dr. Yashashwini Reddy | Sep 8, 2025
Difference Between FDA Form 483 and Warning Letter 1. FDA Form 483 (Inspectional Observations) Issued when: FDA inspectors observe potential violations of the Food, Drug, and Cosmetic Act (FD&C Act) during an inspection. Purpose: To notify company management of...

by Dr. Yashashwini Reddy | Sep 8, 2025
7 Steps for Monitoring Compliance in Pharmaceuticals 1. Establish a Robust Quality Management System (QMS) Build SOPs, policies, and guidelines aligned with FDA, EMA, and ICH standards. Ensure document control and version management. 2. Conduct Regular Internal Audits...

by Dr. Yashashwini Reddy | Sep 8, 2025
1. Be Inspection-Ready Always Keep documentation, equipment, and facilities in a state of compliance at all times. Conduct regular self-inspections and mock audits. Ensure all records are updated, accurate, and readily retrievable. 2. Train Employees on GMP and...







