
by Dr. Yashashwini Reddy | Sep 9, 2025
🧫 Typical Microbiology Concerns in an FDA Inspection 1. Environmental Monitoring (EM) Deficiencies Inadequate EM program for cleanrooms and controlled areas. Failure to establish alert/action limits based on historical data. Poor trending and lack of investigation of...

by Dr. Yashashwini Reddy | Sep 9, 2025
🧾 Improving Quality Through Supplier Audits in Pharmaceuticals Supplier audits are critical in ensuring that raw materials, APIs, packaging components, and outsourced services meet GMP and regulatory expectations. A strong supplier audit program reduces compliance...

by Dr. Yashashwini Reddy | Sep 9, 2025
🧾 Common FDA 483 Observations Related to Cleaning in Pharmaceuticals 1. Inadequate Cleaning Validation Cleaning validation not performed for all product-contact equipment. Worst-case product selection (hardest to clean, most toxic/potent, least soluble) not justified....
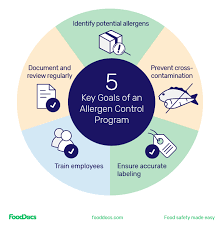
by Dr. Yashashwini Reddy | Sep 9, 2025
🧾 Allergen Control Plan for Pharmaceuticals 1. Risk Assessment Identify allergenic excipients (e.g., lactose, soy lecithin, egg-derived albumin, peanut oil, gluten, gelatin). Assess risk of cross-contamination during manufacturing, packaging, storage, and cleaning....
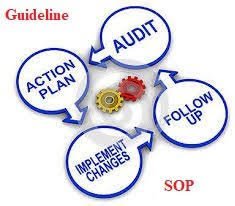
by Dr. Yashashwini Reddy | Sep 9, 2025
✅ Areas to Inspect During Self-Inspection in Pharmaceuticals Personnel & Training Verify staff qualifications, training records, and adherence to GMP practices. Premises & Facilities Check cleanliness, maintenance, pest control, HVAC systems, and environmental...







