
by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 Guidelines for Preparation of Site Master File (SMF) 📑 1. General Information Company name, address, and contact details. Name of parent company (if applicable). Manufacturing activities performed at the site. Products handled (human, veterinary, APIs, biologicals,...

by Dr. Yashashwini Reddy | Sep 10, 2025
💧 Guide to Inspections of High Purity Water Systems 1. System Design & Qualification ✅ Is the system designed with sanitary construction (316L SS, orbital welding, sloped piping, drainability)? ✅ Has the water system undergone DQ, IQ, OQ, and PQ? ✅ Are P&IDs...

by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 Checklist for Internal Audit / Self-Inspection (Defects & Regulatory Compliance) 1. Documentation & Data Integrity ✅ Are records complete, contemporaneous, and accurate (ALCOA+ principles)? ✅ Any overwriting, missing data, or backdated entries? ✅ Are...

by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 GMP Audit Checklist – Storage of Starting Materials 1. Material Receipt & Identification ✅ Are incoming starting materials received against approved suppliers and purchase orders? ✅ Are materials inspected for damage, tampering, contamination, and labeling...
by Dr. Yashashwini Reddy | Sep 10, 2025
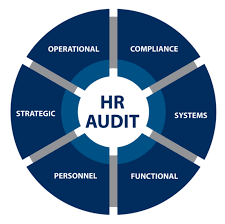
👥 Checklist for Audit in HR / Admin (Pharmaceuticals) 1. Organization & Policies ✅ Organization chart updated and approved. ✅ HR policies documented (recruitment, promotion, disciplinary action). ✅ Job descriptions (JDs) defined, approved, and role-specific. ✅...







