
by Dr. Yashashwini Reddy | Oct 9, 2025
Continuous Manufacturing (CM) is an advanced pharmaceutical production approach where input materials are continuously fed into the system and the processed output (finished product) is continuously removed. Unlike traditional batch manufacturing, which occurs in...
by Dr. Yashashwini Reddy | Oct 9, 2025
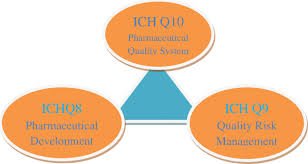
Pharmaceutical development, as described in ICH Q8 (R2), is the process of designing a quality product and its manufacturing process to consistently deliver the intended performance. The goal is to understand how formulation and process variables influence product...

by Dr. Yashashwini Reddy | Sep 2, 2025
🔹 1. Process Optimization Lean Manufacturing (Lean Pharma): Eliminate non-value-added steps, reduce waiting times, optimize material flow. Six Sigma & QbD (Quality by Design): Use statistical tools (DoE, risk assessment) to identify Critical Process Parameters...

by Dr. Yashashwini Reddy | Sep 2, 2025
How to calculate To calculate the capacity of tablet coating pan we need two factors which are given as below, Brimful volume of pan Tablet bulk density Bring full volume is the volume of the water which can be added in the tablet coating pan up to its brim or upper...

by Dr. Yashashwini Reddy | May 1, 2025
Quality by Design (QbD) in Pharmaceuticals: A Detailed Explanation Quality by Design (QbD) is a systematic approach to pharmaceutical development that aims to ensure the quality of a drug product through the design and control of the manufacturing process. This...







