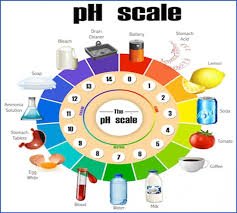
by Dr. Yashashwini Reddy | Aug 10, 2025
pH Value, pH Scale, and Its Measurement (0–14) 1. pH Value Definition: pH is a measure of the hydrogen ion concentration [H+][H⁺][H+] in a solution, indicating its acidity or alkalinity. Formula: 2. pH Scale (0–14) pH Range Nature Example 0–3 Strongly acidic Battery...

by Dr. Yashashwini Reddy | Aug 9, 2025
Working Principle of a pH Meter Definition A pH meter is an analytical instrument used to measure the hydrogen ion concentration ([H⁺]) in a solution, expressing it as pH (potential of hydrogen). Principle A pH meter works on the electrochemical principle of measuring...
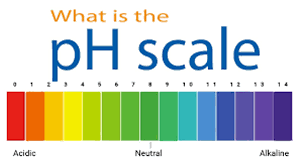
by Dr. Yashashwini Reddy | May 1, 2025
pH Value, pH Scale, and Its Measurement between 0 and 14:- 1. What is pH? pH is a measure of how acidic or basic (alkaline) a solution is. It is a scale used to quantify the concentration of hydrogen ions (H⁺) in a solution. The pH scale ranges from 0 to 14, with: A...

by Dr. Yashashwini Reddy | Apr 29, 2025
The operating principle behind a pH meter is based on the electrochemical measurement of the hydrogen ion concentration (H⁺) in a solution. The pH meter works by measuring the voltage (electromotive force) generated between two electrodes—a glass electrode and a...

by Dr. Yashashwini Reddy | Apr 28, 2025
Accurate pH measurements depend heavily on the condition of your electrode. A neglected or poorly maintained electrode can cause unstable, slow, or incorrect readings. To keep your pH meter electrodes reliable and precise, follow these detailed care guidelines: 1....







