
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish a procedure for regular inspection of utilities (e.g., purified water, compressed air, HVAC system, steam, and gas supply) to ensure compliance with GMP requirements and operational efficiency. 2. Scope This procedure applies to all utility...
by Dr. Yashashwini Reddy | Sep 19, 2025
Water for Injection (WFI) in Pharmaceuticals Definition Water for Injection (WFI) is the highest purity water used in the pharmaceutical industry. It meets pharmacopeial standards (USP, EP, IP, JP) and is free from pyrogens, endotoxins, and microbial contamination....
by Dr. Yashashwini Reddy | Sep 19, 2025
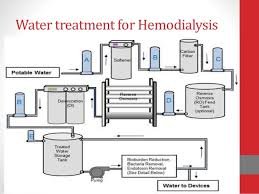
Water for Hemodialysis Definition Water for Hemodialysis is specially treated water used to prepare dialysis fluid (dialysate) and for reprocessing hemodialysis machines and equipment. Since patients are directly exposed to large volumes of water during dialysis...

by Dr. Yashashwini Reddy | Sep 19, 2025
Definition Pure Steam is steam that has been purified to meet stringent quality standards, typically free from non-condensable gases, impurities, chemicals, and microorganisms. It is generated from Purified Water (PW) or Water for Injection (WFI) using a Pure Steam...

by Dr. Yashashwini Reddy | Sep 18, 2025
📌 Types of Water in Pharmaceuticals, Usage, and Preparation Techniques 🔹 1. Potable Water (Drinking Water) Usage: Base feed for preparation of Purified Water, WFI, and Clean Steam. Used for general cleaning (non-product contact). Preparation Technique: Obtained from...







