by Dr. Yashashwini Reddy | Aug 28, 2025
1.0 Purpose To establish a procedure for performing finger dab (finger imprint) tests for monitoring personnel hygiene practices and aseptic conditions in classified areas. 2.0 Scope This SOP applies to all personnel working in aseptic and controlled areas (Grades A,...

by Dr. Yashashwini Reddy | Aug 28, 2025
1.0 Purpose To define the procedure for the preparation of disinfectant solutions used in pharmaceutical/biotechnology facilities for cleaning and sanitization purposes. 2.0 Scope This SOP applies to all personnel involved in the preparation, dilution, and labeling of...

by Dr. Yashashwini Reddy | Aug 28, 2025
1. Purpose To define a standardized procedure to evaluate and verify the antimicrobial effectiveness of disinfectants and sporicidal agents used for routine cleaning and disinfection of classified and non-classified areas, equipment surfaces, and utilities in the...

by Dr. Yashashwini Reddy | Aug 18, 2025
Writing Effective SOPs in Pharmaceuticals Standard Operating Procedures (SOPs) are the backbone of pharmaceutical operations, ensuring consistency, compliance, and quality across all processes. An effective SOP must be clear, concise, and compliant with GMP (Good...
by Dr. Yashashwini Reddy | Aug 18, 2025
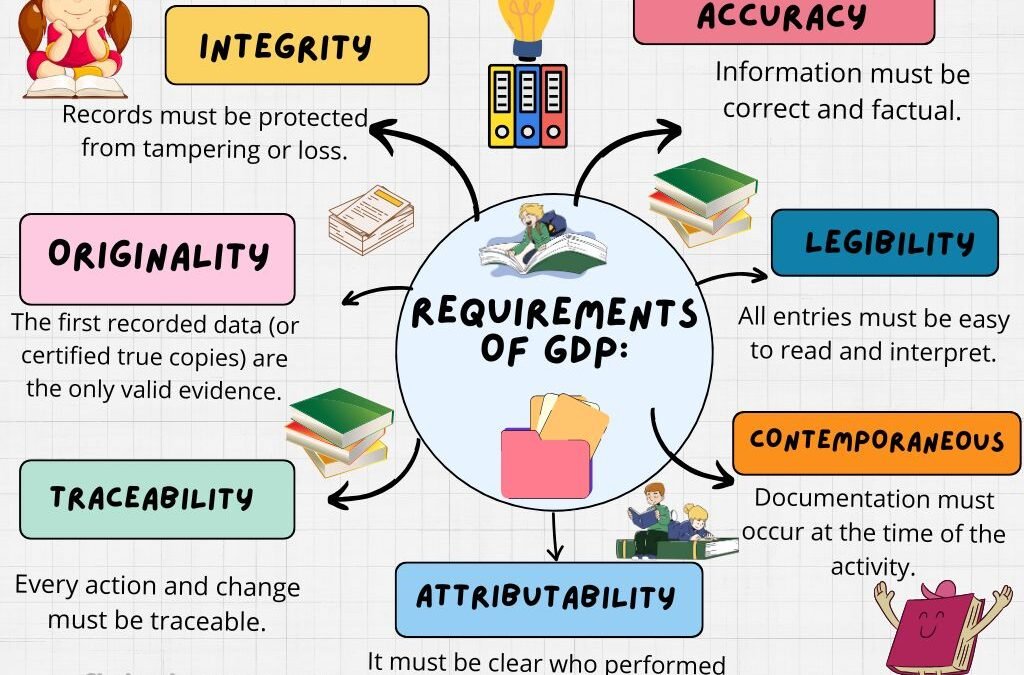
Requirements for Good Documentation Practice (GDP) Legibility All entries should be clear, readable, and permanent (no pencil or erasable ink). Accuracy Data should reflect the actual observation, measurement, or action without manipulation. Contemporaneous Recording...







